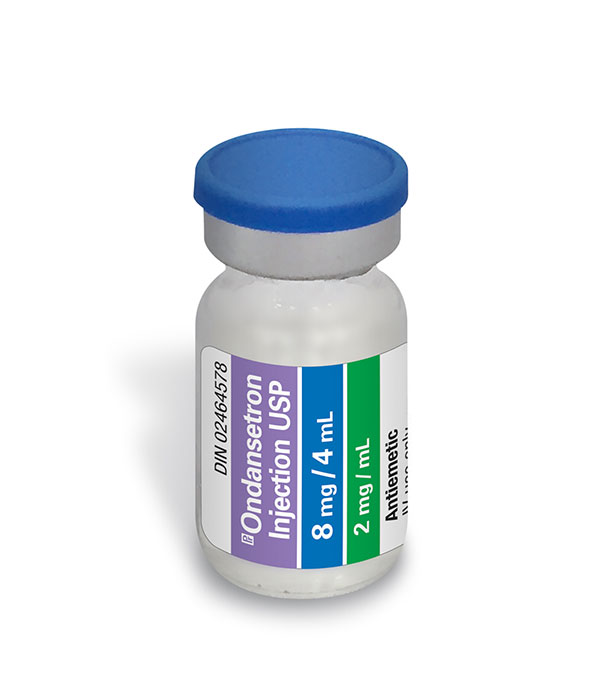
Ondansetron Injection USP
| Generic Name | Ondansetron Injection USP |
|---|---|
| Brand Reference | Zofran® |
| DIN | 02464578 |
| GTIN | 834324002200 |
| Strength | 2 mg / mL |
| Fill size | 4 mL |
| Format | Vial |
| Pack Size | 10 |
| Therapeutic Class | Antiemetic |
| Product Insert | Click Here |
| Product Monograph | Click Here |